Abstract
Treatment with high dose melphalan (HDM) and autologous stem cell transplantation (ASCT) improves progression free survival (PFS) and overall survival (OS) of myeloma patients and is a standard therapy for eligible patients. Furthermore, the use of maintenance therapy after ASCT is beneficial. However, a subset of patients relapses early after HDM, and there is a paucity of data about their outcomes and characteristics, especially regarding their identification at diagnosis or early in the course of the disease. The aim of the current study was to describe the characteristics, the management and outcomes of patients relapsing within 12 months from HDM.
The study included 297 consecutive patients treated in the Department of Clinical Therapeutics, Athens, Greece, which received HDM-ASCT as part of first line therapy from 1995 until June 2016. Median age was 55 years (range 22-67); 38% were ISS-1, 31% ISS-2 and 30% ISS-3. Induction was bortezomib-based in 47%, thalidomide-based in 13%, lenalidomide-based in 6%, high dose dexamethasone-based in 25% and contained both bortezomib and an IMiD in 9%. After ASCT, 35% received consolidation and 30% maintenance.
Median follow up for all patients is 5 years, 183 (62%) have relapsed, the median PFS post HDM was 36 months and 43 (13.5%) patients have progressed within <12 months post HDM. In univariate analysis, the baseline LDH ≥250 IU/L (37% vs 15%, p<0.001), eGFR <30 ml/min (22% vs 12%, p=0.055), hypercalcemia (26% vs 16%, p=0.069) and high-risk cytogenetics (available in 138 patients) (39% vs 23%, p=0.084) were associated with early relapse. Response to induction therapy was not predictive of early relapse but less than sCR/CR post HDM (day 100) was associated with early progression (p=0.1). The use of consolidation therapy post HDM was associated with lower rates of early relapses (4% vs 14% for maintenance and 26% for no post-HDM therapy, p<0.001). In multivariate analysis, LDH ≥250 IU/L (HR: 3.4, p=0.01) and hypercalcemia (HR: 3, p=0.006) at diagnosis increased while the use of consolidation reduced the risk of early relapse (HR: 0.23, p=0.012). Due to the limited number of patients with cytogenetics this parameter was not included in the multivariate model.
Baseline characteristics that were associated with PFS post HDM included high-risk cytogenetics (HR: 2.1, p=0.003), ISS-3 (HR: 1.4, p=0.03), hemoglobin <10 g/dl (HR: 1.6, p=0.001), hypercalcemia (HR: 1.6, p=0.014) and achievement of sCR/CR after HDM (HR: 0.8 vs VGPR, p=0.18 and HR: 0.67 vs PR, p=0.06). The use of maintenance therapy (HR: 0.54, p=0.01) was also associated with better PFS. In multivariate analysis, the use of maintenance therapy (HR: 0.58, p=0.032) and sCR/CR post HDM (HR: 0.64 vs PR, p=0.06 and HR: 0.9 vs VGPR, p=0.17) were independently associated with PFS. In further analysis, PFS to 2nd line therapy was 14 months for all patients, but only 5 months for those with an early relapse vs 19 months for the other patients (P<0.001). Median PFS2 was 15.5 months for patients with a relapse <12 months post HDM vs 57% at 5 years for the other patients (p<0.001).
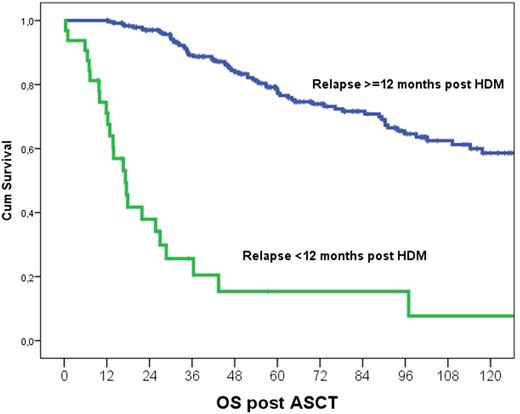
The 5-years OS is 67%, but, median post HDM OS was 18 months for those with an early relapse vs 71% at 5 years for those with post HDM PFS ≥ 12 months (p<0.001; Figure). In univariate analysis prognostic factors for OS after HDM included high risk cytogenetics (HR: 3, p<0.001), ISS-3 (HR: 2.2, p<0.001), hypercalcemia (HR: 2.8, p<0.001), anemia (HR:1.9, p=0.001), LDH ≥250 IU/L (HR: 1.7, p=0.054) and sCR/CR after HDM (HR: 0.7, p=0.082), use of maintenance (HR: 0.5, p=0.014) or consolidation (HR: 0.61, p=0.017) after HDM. In multivariate analysis PFS post ASCT <12 months (HR: 14, p<0.001) was the most important prognostic factor; other factors included post-HDM sCR/CR (HR: 0.48 vs PR, p=0.021 and HR: 0.54 vs VGPR, p=0.054), hypercalcemia at diagnosis (HR:2.1, p=0.005) but not maintenance (p=0.1) or consolidation therapy (p=0.54) post ASCT.
In conclusion, 13% of patients with newly diagnosed MM that receive HDM at first remission relapse within 12 months after ASCT and they have very poor outcomes. These patients should be considered as an ultra high-risk group that urgently needs more effective therapies and innovative approaches through targeted clinical trials. The use of consolidation in patients at high risk for early relapse, such as those with high LDH and hypercalcemia at diagnosis, may reduce early relapses, and maintenance may prolong PFS.
Kastritis: Janssen: Honoraria, Research Funding; Gebesis/Celgene: Honoraria; Amgen: Honoraria, Research Funding; Takeda: Honoraria. Terpos: Abbvie: Honoraria; BMS: Honoraria; Amgen: Honoraria, Other: SC member, Research Funding; Janssen: Honoraria, Research Funding; Genesis/Celgene: Honoraria, Other: DMC member, Research Funding; Takeda: Honoraria, Other: SC member; GSK: Honoraria. Dimopoulos: Novartis: Consultancy, Honoraria; Genesis Pharma: Research Funding; Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology: Consultancy, Honoraria, Other: Advisory Committee: Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Onyx Pharmaceuticals, an Amgen subsidiary, Takeda Oncology.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal